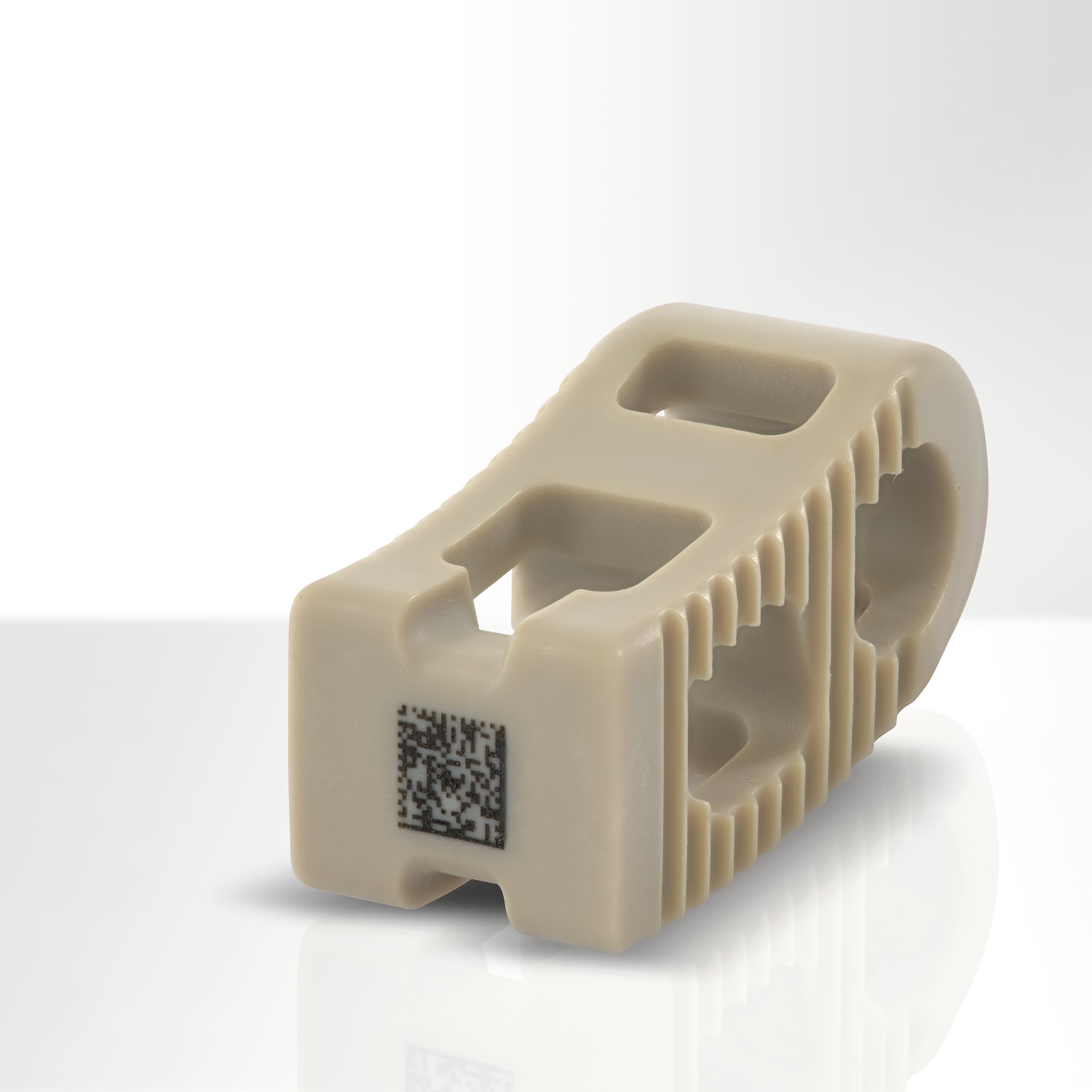
Severe criticism against manufacturers of medical devices and related supervision processes occurred in November 2018 and questioned the reliability of implants. In this context, FOBA considers UDI-codes, firmly applied on medical implants, to be a key-factor for safe traceability of implants.
Selmsdorf, February 2019 - Severe criticism against manufacturers of medical devices and related supervision processes occurred in November 2018, when European journalists questioned the reliability of implants and their possible effects on human health. The German inter-trade organization BVMed put this into perspective and additionally pleaded for an official nationwide implant database. FOBA encourages this approach and considers UDI-codes, firmly applied on medical implants, to be a key-factor for safe traceability of implants.
Laser marking on products from the medical sector, like UDI-codes and other marks applied directly on the part, make every single item traceable throughout its entire product life cycle from manufacturing to patient. This is of importance especially for the safety of patients and to enhance product quality.
An implant database, like the already existing EPRD (Endoprothesenregister Deutschland) indicates quality standards and can prevent malfunctions of an endoprosthesis. Patient safety and product quality can significantly be improved if manufacturers and users (medical professionals, hospitals, patients etc.) provide information and report incidents consistently. A UDI (Unique Device Identification) makes every single implant explicitly traceable.
A UDI mark, applied permanently on a medical product, is presently only mandatory for reusable and multi-reprocessed parts like surgical instruments. But also on implants, although often delivered sterile, a resistant and biocompatible UDI mark is of benefit for traceability when it comes to possible revision surgery in the case of malfunction.
Additionally the increasing digitalization in the health sector raises the importance of machine-readable UDI-codes directly on medical products. More and more users, e.g. in the complex environment of a hospital, request direct part marking with codes and other indicators for logistical reasons.
According to the 2017 annual report of Endoprothesenregister Deutschland (EPRD) about 63 percent of all endoprosthestic hip and knee joint surgeries in Germany have already been registered. A growth to over one million registrations is being estimated until the end of 2018, the aim according to the EPRD is a fully registration of all implantations. Despite its comparably short term of existence the EPRD is one of the worldwide largest registers (see EPRD annual report 2017).
Hospitals and medical professional are requested to contribute to the EPRD even more rigorously with the aim to track the lifespan (duration in the body) of the implants as completely as possible. German federal health minister Jens Spahn has recently presented a draft law for an official implant register, based on the already existing EPRD.
Whereas the EPRD basically covers all implantations and possible subsequent revision surgeries, the general medical devices law also requires a compulsory registration for all incidents in the sense of product defects that actually threaten or are a potential threat for human health. The BVMed e.V. (Bundesverband Medizintechnologie) states that most incidents so far have been reported by the manufacturers and distributors. This shows that manufacturers and distributors are willing to account for the safety and quality of medical products.
The manufacturers and distributors of medical devices are currently challenged to adapt their processes to the latest requirements of the European MDR, becoming mandatory as of 2020. There seems to be a tremendous need for consultation, especially regarding part marking with law-confirming appropriate UDI-codes. “We can support all medical device manufacturers in implementing a compliant marking process which also translates into providing the best possible safety for patients”, says Christian Söhner, FOBAs Global Vertical Manager Medical.
FOBA’s vision-based laser marking systems make sure that all kinds of marks or (bar-)codes are applied in highest quality and permanency on any substrate. Manufacturers who use FOBA’s M-series laser marking machines benefit from a universal and comprehensive solution for UDI-code marking that also includes a pre- and post-mark validation of parts and marks.
FOBA Laser Marking + Engraving
www.fobalaser.com