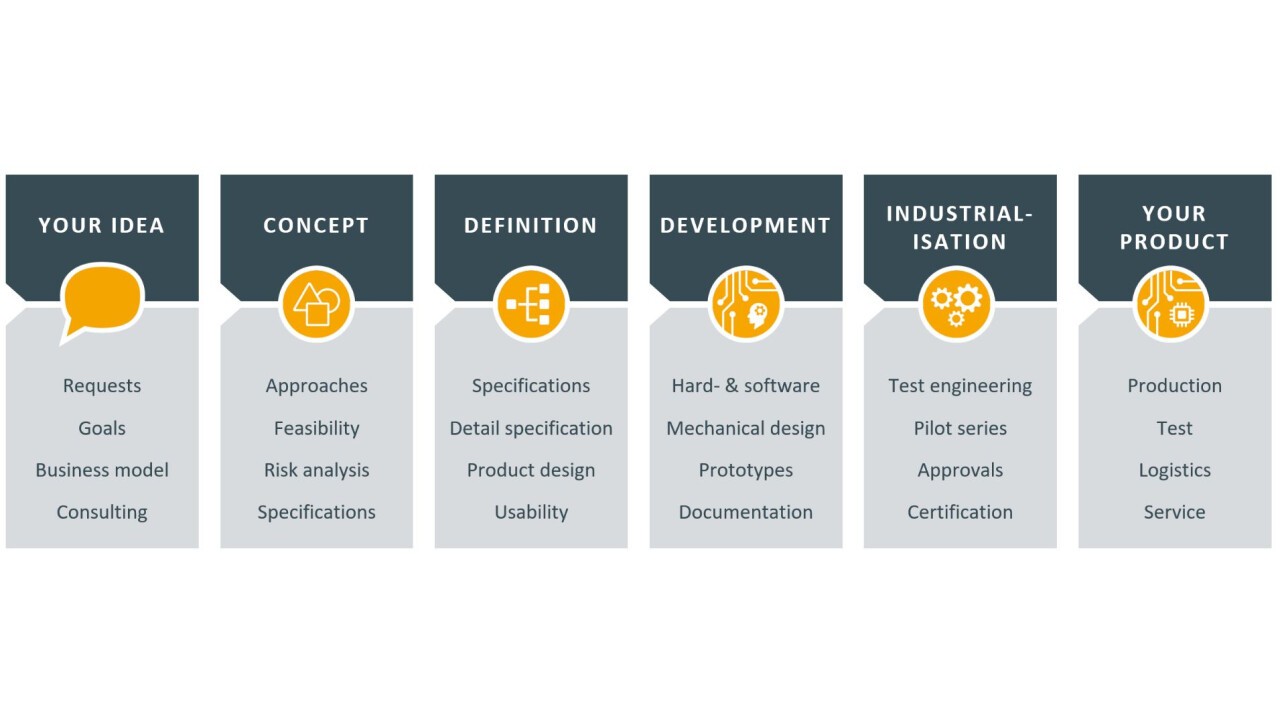
Creativity and conformity to standards - a contradiction in medical technology? Are you looking for a discussion on topics related to the development of electronic assemblies or devices? Our experts will be there for you!
Do you have to meet international requirements? We support you with our ISO 13485-certified development! Our competences in engineering are your advantage:
- Software & hardware development with device design
- Industrial design and usability (UI/UX design)
- Agile project management including subcontractor integration
- Certifications such as MDR, MDSAP, FDA
- Management of the entire product cycle (parts management, redesign)
Afterwards, we are happy to offer you the production and logistics of your electronic assemblies.