ICP-MS is an extremely sensitive technique that allows simultaneous quantification of 70 elements down to trace levels in the μg/L or sub-μg/L range, and can therefore handle a large variety of analytical challenges.
Inorganic chemical analysis plays an important role in guaranteeing the safety and performance of countless materials and products. In particular, medical devices in contact with the human body must comply with strict maximum limits of potentially harmful elements such as heavy metals. ICP-MS (inductively coupled plasma-mass spectrometry) is an extremely sensitive technique that allows simultaneous quantification of 70 elements down to trace levels in the μg/L or sub-μg/L range, and can therefore handle a large variety of analytical challenges.
At RMS Foundation, we offer services in various areas including the MedTech, pharma, chemical and machine industry. ICP-MS can be applied to most engineering materials including metallic alloys, ceramics, polymers as well as natural materials and liquid samples, if necessary with a chemical digestion process prior to analysis.
The following examples highlight ICP-MS analyses that were tailored to meet medical device regulatory requirements. The methods were material-specifically validated in internal studies as well as round robin comparisons in order to guarantee accurate results.
Leachables and extractables in compliance with ISO 10993: As part of the biological evaluation of medical devices, products must be tested for surface contaminants in «leachable» and «extractable» studies. RMS Foundation offers incubation tests according to ISO 10993 followed by ICP-MS analysis of more than 60 trace elements released into solution.
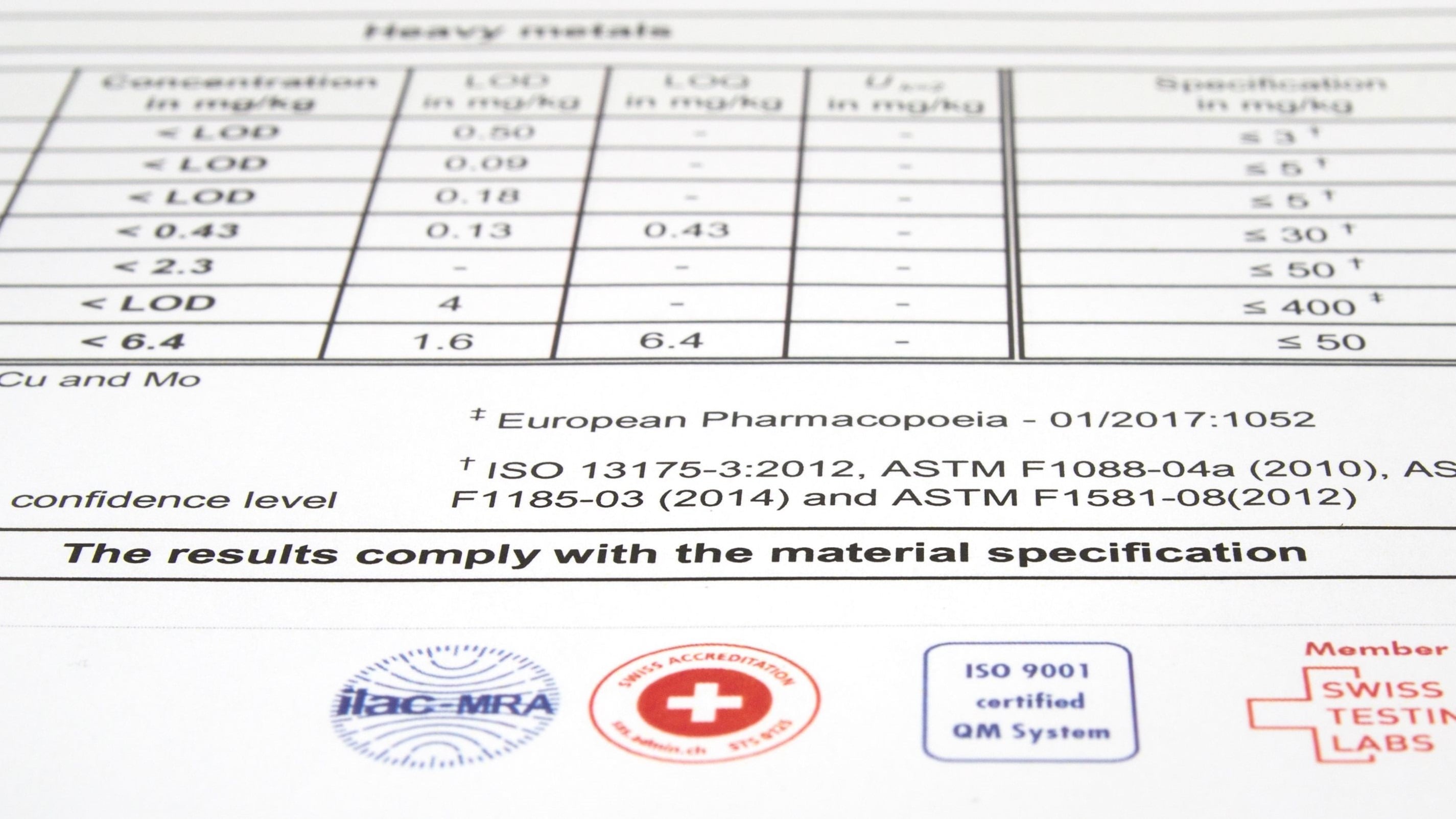
Trace elements in bone graft substitutes: Implantable calcium phosphate materials are tested for heavy metals such as arsenic, cadmium, mercury and lead according to the maximum allowable limits defined in ISO 13175-3, ASTM F1088, F1185 and F1581, along with a simultaneous screening for 50 additional elements.
Dissolution testing of bone graft substitutes: degradable calcium phosphates are characterised in an in vitro dissolution test recording the Ca release and pH changes according to ISO 13175-3.