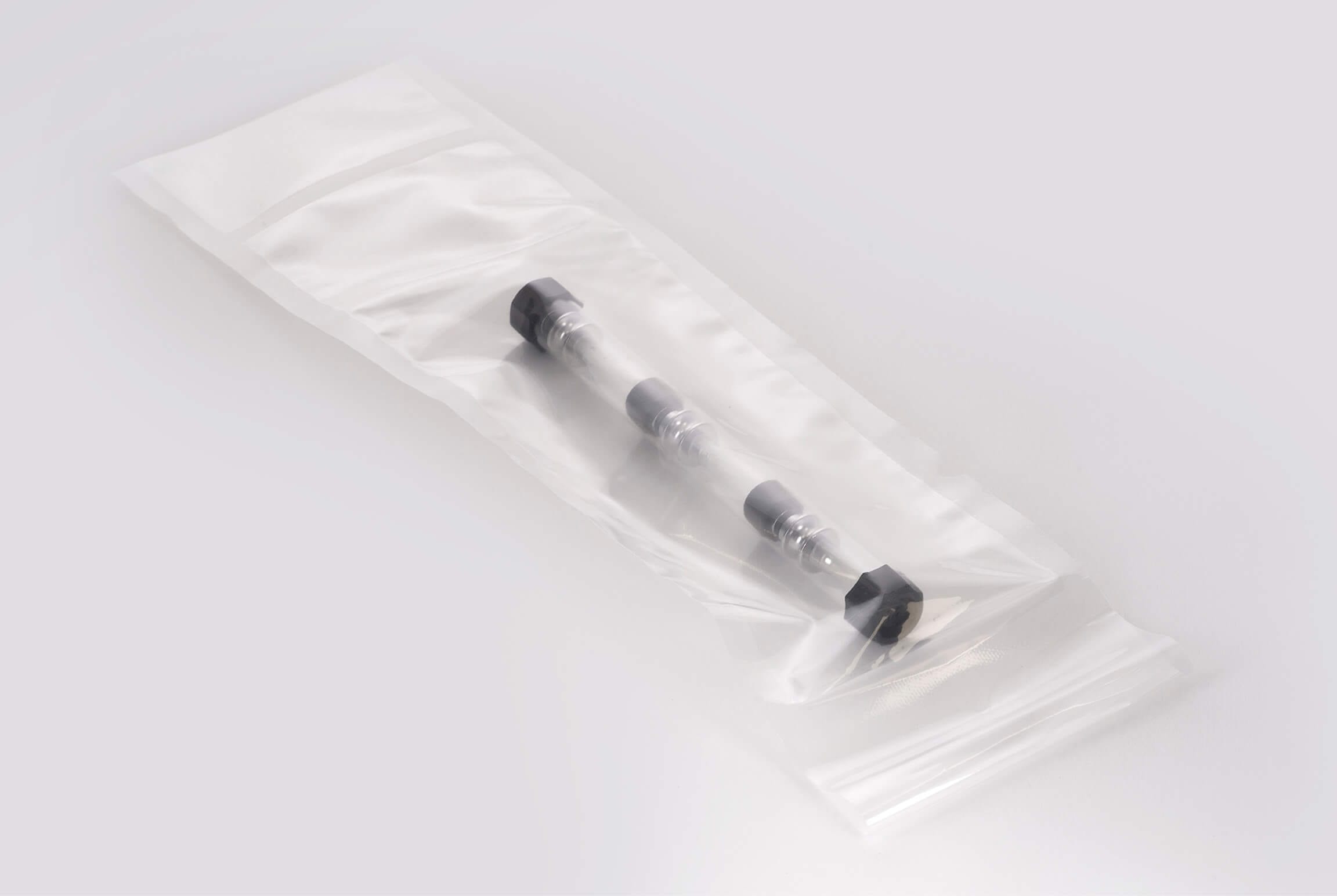
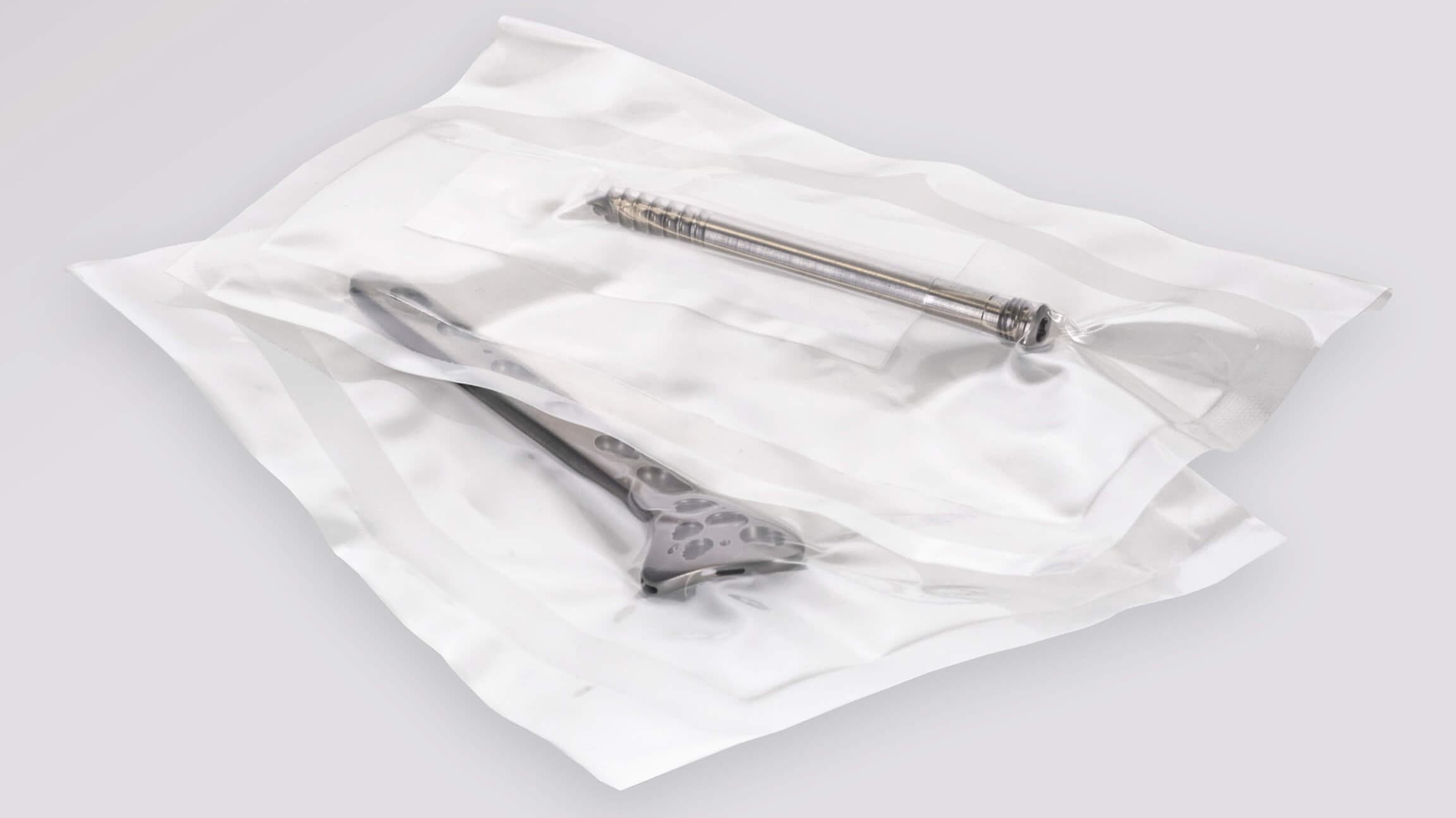
PA/PE pouches have very high mechanical strength and are ideal for vacuum packaging of heavy medical devices with sharp and rough surfaces. The material is suitable for gamma sterilisation. Also available as a pre-validated packaging solution.
PA/PE pouches with peel – flexible packaging for medical device
Perfect fixing of the medical devices through vacuum packaging
Our PA/PE pouch, consisting of a transparent PA/PE composite combination, is characterised by its outstanding peel properties for an aseptic opening. The packaging also affords additional protection for your devices, as these are fixed ideally in the pouch by vacuum packaging. In cooperation with the Swiss machine manufacturer VC999 Medical, we are able to offer you a completely validated packaging process, which includes both: validation of the C6 Medical packaging machine by VC999 and validation of the PA/PE pouch. You can take over this validated process directly and thus facilitate fast production of flexible and cost-optimised packaging solutions.
Features
• Flexible film with good sealing and peel properties
• Device fixing by means of vacuum packaging
• High mechanical stability
• Smooth and fibre-free peel
• Complies with the requirements of DIN EN ISO 11607
• Shelf life of 10 years (based on the packaged product)
Applications
The PA/PE pouch is a flexible packaging for different sizes of medical device such as:
• Heavy devices, with rough and sharp-edged surfaces
• Implants
• Surgical instruments
Sterilisation methods
• gamma sterilisation
Substances
PA/PE pouches do not contain any substances such as latex, PVC, bisphenol A, colophony (rosin), TSE/BSE (transmissible/bovine spongiform encephalopathy) risk materials, SVHC or phthalates.
Safety and quality in medical packaging – Made in Germany
The quality of all our products is intimately connected with the continuously rising demands on hygiene and medical technology. Therefore, all our products meet or exceed European and international standards. The management systems of Vereinigte Papierwarenfabriken GmbH has been assessed and certified as meeting the requirements of EN ISO 13485:2012 for design, development, manufacturing and sales
of medical packaging systems. We maintain our leadership with a department for development and applications, a branch for development and production of indicators,
comprehensive quality management and consistent in-process control.