Like all medical device manufacturers, Tecan is subject to a growing number of regulations. Learn how Tecan reduced its effort by up to 80 % with a modular documentation approach.
Empowering life sciences and diagnostics
As a global provider of automated laboratory instruments and solutions, Tecan develops systems and components that help people working in clinical diagnostics, basic and translational research and drug discovery.
In particular, Tecan develops, produces, markets and supports automated workflow solutions that empower laboratories to achieve more.
Modular Design Control Documentation
Like all medical device manufacturers, Tecan is subjected to a growing amount of regulations demanding an ever-increasing amount of documentation to be created and maintained.
A large part of the challenge consists in getting the Design Control Documentation in place for market submission. As new products and product versions are brought to the market, the manufacturer is facing a growing administrative effort to keep the Development Documentation consistent and up-to-date.
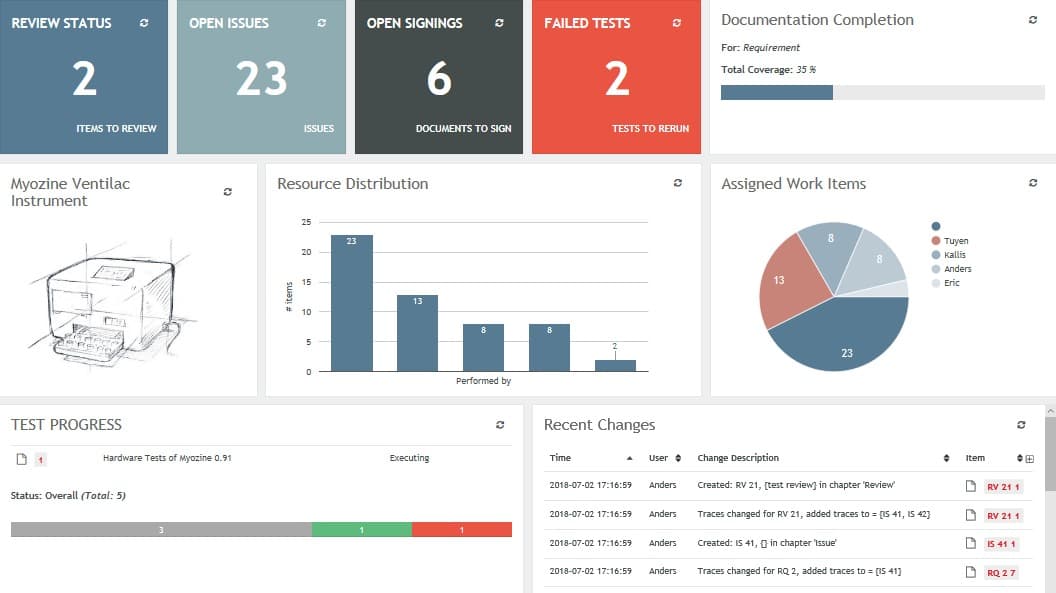
With the objective of digitizing its current operations, Tecan decided to bring in Aligned Elements to manage of their Design Control Documentation. Aligned Elements is a Medical Device ALM specifically developed to efficiently capture, trace and manage Design Control Items in a single repository, including requirements, specifications, risks and verifications.
Document once and re-use
Aligned Elements permitted documenting individual Tecan Instrument modules in separate documentation packages, where each module consists of a set of specifications, risk assessments and verifications inferred from a module; all including an over-all traceability and with a complete, encapsulated audit trail.
By separating product specific Design Control data from the module Design Control data in separate packages, Aligned Elements can “link” the product and the module DHF data and set traces between the design control items across package boundaries. A single module documentation package can thus be used in a multitude of products.
Faster with less effort
The modular documentation approach permits the manufacturer to reach a significant degree of reuse of existing Design Control data, reaching levels of up to 80% reuse. For new products, Tecan manages to set up a large part of the product documentation with in hours by simply referring and tracing to DHF content in existing modules.
"We reuse an extensive set of existing Design Control data which has already been reviewed, verified and approved, without disrupting the traceability and change control for existing product documentation. We can now generate the documentation for a new version in a single day which previously took a week to set up.”- Phil Costello, Senior Software Manager, Tecan