Showcase Swiss Medtech Expo 2021
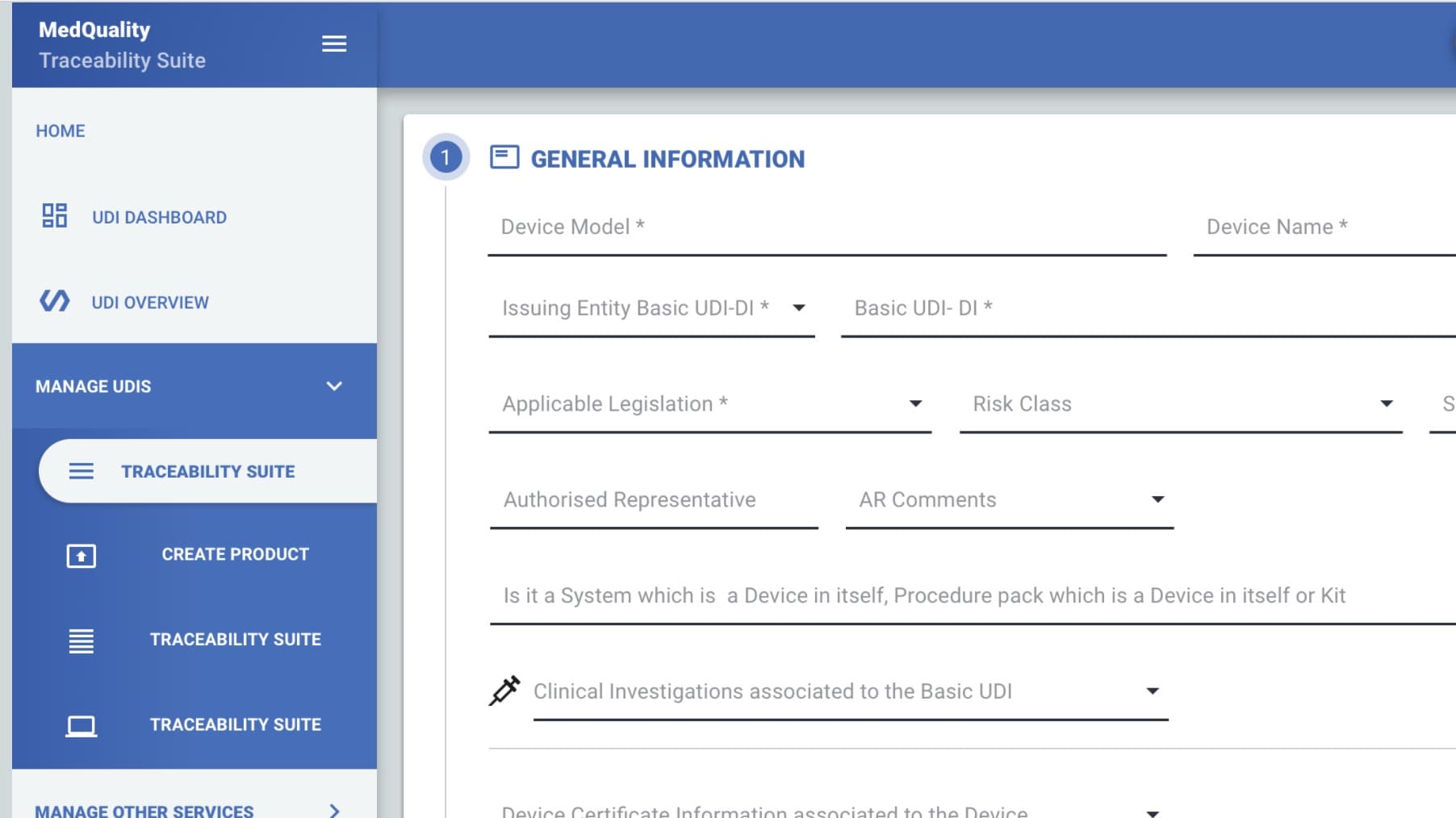
UDI-EUDAMED Tool - Fullfil Eudamed obbligations in one go
The MQ Traceability Suite guided procedure allows the manufacturer to fill in all information required by EUDAMED and therefore be ready to communicate them without effort.